Author: Barry A. Singer, MDComplete author affiliations and disclosures are at the end of this activity.
http://www.medscape.com/viewprogram/4281_pnt
Release Date: June 30, 2005; Valid for credit through June 30, 2006
Target Audience
This activity is intended for general neurologists, primary care practitioners, nurse practitioners, physician assistants, and nurses.
Goal
The goals of this activity are to improve the diagnosis of multiple sclerosis and its treatment, focusing on relapsing-remitting disease. Specific topics discussed are the following: (1) the use of MRI in diagnosis and monitoring disease activity, (2) assessing treatment failure, (3) switching primary therapy, (4) add-on therapy, and (5) new treatments in development.
Learning Objectives
Upon completion of this activity, participants will be able to:
- Differentiate between the types of MS.
- Review the use of MRI in initial diagnosis and in monitoring disease activity.
- Assess treatment failure.
- Evaluate options for switching primary therapy.
- Examine add-on therapies and new products currently in development.
Credits Available
Physicians - up to 1.0 AMA PRA Category 1 continuing physician education credits ;
Nurses - up to 1.2 ANCC continuing nurse education contact hours
All other healthcare professionals completing continuing education credit for this activity will be issued a certificate of participation.
Participants should claim only the number of hours actually spent in completing the educational activity.
Accreditation Statements
For Physicians

Medscape is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Medscape designates this educational activity for a maximum of 1.0 Category 1 credit(s) toward the AMA Physician's Recognition Award. Each physician should claim only those credits that reflect the time he/she actually spent in the activity.

For questions regarding the content of this activity, contact the accredited provider for this CME/CE activity:
CME@webmd.net. For technical assistance, contact
CME@webmd.net.
For Nurses

This Activity is sponsored by Nurse Practitioner Alternatives, Inc. as a provider of continuing education in nursing through the Maryland Nurses Association which is accredited as an approver of continuing education in nursing by the American Nurses Credentialing Center's Commission on Accreditation.
Approved for 1.2 contact hour(s) of continuing education for RNs, LPNs, LVNs and NPs. This program is coprovided with Nurse Practitioner Alternatives, Inc., (NPA) and Medscape. NPA is accredited as a provider of continuing nursing education by the Maryland Nurses Association, an accredited approver by the American Nurses Credentialing Center's Commission on Accreditation.
Provider Number: PN04-2-910-809

For questions regarding the content of this activity, contact the accredited provider for this CME/CE activity:
info@npedu.com. For technical assistance, contact
CME@webmd.net.
Instructions for Participation and Credit
There are no fees for participating in or receiving credit for this online educational activity. For information on applicability and acceptance of continuing education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity online during the valid credit period that is noted on the title page.
Follow these steps to earn CME/CE credit*:
- Read the target audience, learning objectives, and author disclosures.
- Study the educational content online or printed out.
- Online, choose the best answer to each test question. To receive a certificate, you must receive a passing score as designated at the top of the test. Medscape encourages you to complete the Activity Evaluation to provide feedback for future programming.
You may now view or print the certificate from your CME/CE Tracker. You may print the certificate but you cannot alter it. Credits will be tallied in your CME/CE Tracker and archived for 5 years; at any point within this time period you can print out the tally as well as the certificates by accessing "Edit Your Profile" at the top of your Medscape homepage.
*The credit that you receive is based on your user profile.
This activity is supported by an independent educational grant from Serono, Pfizer.

Legal Disclaimer
The material presented here does not necessarily reflect the views of Medscape or companies that support educational programming on www.medscape.com. These materials may discuss therapeutic products that have not been approved by the US Food and Drug Administration and off-label uses of approved products. A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Copyright © 2005 Medscape.
Contents of This CME/CE Activity
- Advances in MS: A Case-Based Approach to Management
Introduction
Case Presentation
Classification of the Patient's Clinical Course
Factors Associated With an Increased Likelihood of Disease Progression
Assessment of Treatment Failure
Factors to Consider When Assessing Treatment Failure
Evidence-Based Data for Switching to Another Interferon Medication
Evidence-Based Data for Switching to Glatiramer Acetate
Evidence-Based Data for Switching to Mitoxantrone
Evidence for Preventing Progression of Disability Over the Long Term With Treatment
Add-on Therapies to US Food and Drug Administration-Approved Immunomodulatory Agents for Relapsing-Remitting Disease
Potential New Treatment Options for This Patient on the Horizon
Conclusion
References
Advances in MS: A Case-Based Approach to Management
Introduction
Multiple sclerosis (MS) is an autoimmune disease that targets the myelin of the brain, spinal cord, and optic nerves. This immune attack results in demyelination and, potentially, axonal injury. The inciting trigger that activates this immune response is unknown. Potential causes may be a combination of genetics and environmental exposures to pathogens, such as viruses.
The disease affects young adults with the mean age of onset at about 30 years. Two thirds of people with MS are women. Eighty-five percent of patients initially have a relapsing-remitting course in which acute exacerbations are generally followed by neurologic improvements.[1] Over time, disability accrues from residual neurologic impairment from relapses and/or a progressive course of worsening function. Physical disability, such as loss of ambulation or vision, can severely restrict the lives of individuals with MS. Cognitive disabilities, such as short-term memory loss and impairment in multitasking, can result in loss of employment with marked psychological and socioeconomic consequences. Natural history studies have demonstrated that 90% of patients have developed progressive disease after 25 years without treatment.[2]
The introduction of immunomodulatory treatment options has begun to alter the clinical course for people living with relapsing-remitting MS. Beta-interferon and glatiramer acetate injections have been shown to reduce relapse rates and reduce disability progression.[3-6] Hospitalizations and absences from work due to relapses can significantly disrupt an individual's life. Furthermore, some relapses can lead to residual disability.[7] Interferon beta-1a and mitoxantrone have also been shown to reduce the probability of disease progression to a statistically significant degree in relapsing-remitting MS patients.[4,5,8]
Case Presentation
A 29-year-old, right-handed man presents for evaluation of worsening balance. Three years ago, he developed intermittent right face numbness for 2 months. Two years ago, he developed right leg tingling for approximately 3 months. He also noted that his balance was intermittently impaired at that time. A magnetic resonance imaging (MRI) scan of the brain, obtained 2 years ago, demonstrated 22 enhancing lesions and 29 T2-hyperintense lesions (Figure 1).
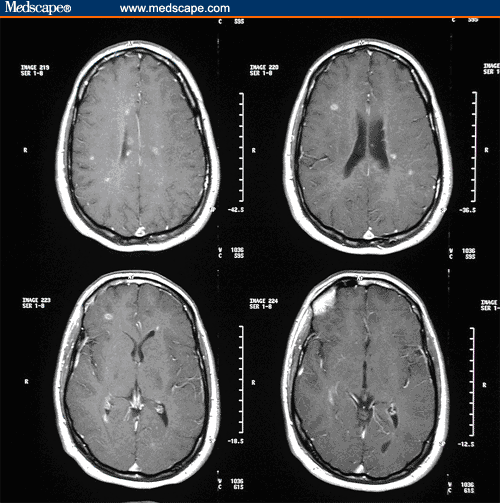
Figure 1. Contrast-enhancing T1 lesions prior to treatment initiation.
No lumbar puncture was performed. At that time, interferon beta-1a 30 mcg intramuscularly (IM) was started on a weekly basis.
One and a half years ago, he developed double vision that lasted for 2 weeks; the images appeared horizontally displaced. Currently, his balance is poor. He must hold onto walls and falls as many as 10 times per month. His legs feel strong, but are stiff. Both his feet tingle. He describes a sense of urinary urgency with a frequency of once an hour. At times, his bladder does not seem to empty completely. He also reports that ejaculation is delayed. He developed depression that has now resolved with fluoxetine. Fatigue had disrupted work and home life, but has now significantly diminished on modafinil. He denies experiencing vertigo, tinnitus, hearing loss, or constipation. His short-term memory has been only minimally impaired, and multitasking ability has been excellent.
His medical history is otherwise unremarkable. He has had no previous surgeries. He is currently taking IM interferon beta-1a 30 mcg weekly, fluoxetine 40 mg daily, and modafinil 200 mg daily, and he denies having medication allergies. He is a nonsmoker and he occasionally drinks alcohol. His parents are both healthy with no significant family history of neurologic illness.
On neurologic examination, he is alert and fully oriented. His speech is fluent without dysarthria. Past presidents are intact to Reagan. "World" spelled backward is correct. Serial 7's are intact to 86. Immediate object recall is 3 out of 3, but only 1 out of 3 in 5 minutes with and without cues. His visual fields are full and his visual acuity is 20/20 bilaterally without correction. Pupils are equal, round, and reactive to light. He has no papilledema or pallor. Extraocular movements are full. His face is symmetric with normal sensation. His hearing is intact and his palate is upgoing bilaterally. Sternocleidomastoid and trapezius strength are intact. His tongue is midline.
His motor exam is notable for moderate spasticity bilaterally in the legs. Strength resistance testing is 5 of 5 in all extremities without a pronator drift. Light touch, pinprick, and proprioception are normal. Reduced vibratory sensation is noted in the left leg and distal to the midshin on the right. Reflexes are normal in the upper extremities, but sustained clonus is present at the knees and ankles bilaterally. Bilateral Babinski's are present. There is no finger-to-nose dysmetria. Heel-to-shin dysmetria is moderate on the right and mild on the left. His gait is severely spastic. Romberg testing is negative and he is able to tandem walk.
A follow-up MRI scan of the brain with gadolinium demonstrates only 1 enhancing lesion in the left occipital lobe. An interval increase in T2 lesions from 29 to 40 occurred. Fluid-attenuated inversion-recovery (FLAIR) axial and sagittal images 2 years after treatment are shown in Figures 2 and 3, respectively.
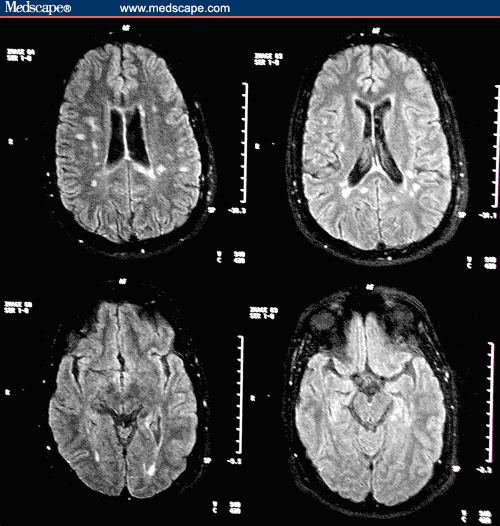
Figure 2. Fluid-attenuated inversion-recovery axial images 2 years after treatment with intramuscular interferon beta-1a 30 mcg weekly.
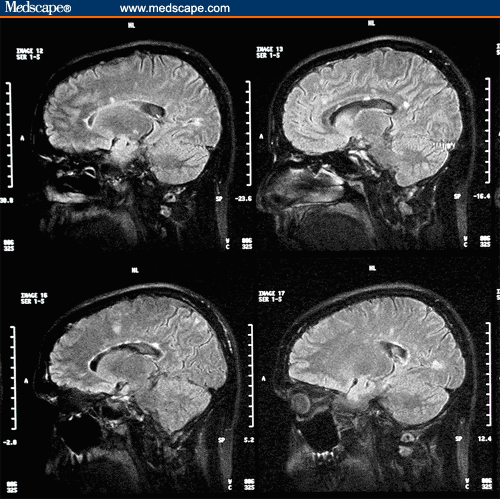
Figure 3. Fluid-attenuated inversion-recovery sagittal images 2 years after treatment with intramuscular interferon beta-1a 30 mcg weekly. Presence of callosal lesions noted.
Classification of the Patient's Clinical Course
Relapsing-remitting disease is the best description for this patient's clinical course. Eighty-five percent of patients initially have this type of MS.[1] Relapsing-remitting disease is characterized by clearly defined relapses and followed by partial or complete recovery periods free of disease progression. Relapses experienced by patients can clearly result in residual disability. In one study by Lublin and colleagues,[7] worsening disability 3 months after relapses was observed in pooled placebo patients from pivotal clinical trials. Thirty percent of placebo patients progressed 1 point and 41% progressed 0.5 points in the Expanded Disability Status Scale (EDSS) 3 months after a relapse.
A secondary progressive course generally implies a steady progressive decline in function. Early in the secondary progressive stage, patients can still have superimposed relapses in addition to the continuous downward slope of increasing disability. Although this patient is at high risk for secondary progressive disease with time, his clinical course is more consistent with relapses with residual disability after each attack.
Fifteen percent of patients have primary progressive MS. In this clinical course, symptoms, such as leg weakness, gradually worsen over years without the presence of relapses.[1]
Factors Associated With an Increased Likelihood of Disease Progression
Brainstem and spinal cord demyelination are unfavorable predictors of disease course. Horizontal diplopia and ataxia suggest pontine involvement. Bilateral leg stiffness, bilateral foot tingling, bladder dysfunction, and sexual dysfunction may be indicative of myelopathy. The patient's examination supports spinal cord involvement with bilateral leg spasticity, vibratory loss, dysmetria, sustained clonus, and Babinski signs.
Together, these observations suggest an increased likelihood of disease progression. An important consideration is that men tend to fare worse in terms of disease progression. This patient is also at higher risk because he experienced 3 relapses in his first 2 years.[9]
Assessment of Treatment Failure
Clinically, this patient experienced marked worsening of his disability despite being on interferon beta-1a 30 mcg weekly. In 2 years, he developed ataxia, spasticity, foot paresthesias, bladder dysfunction, fatigue, and depression. Therefore, his response to this immunomodulatory regimen could be considered a treatment failure. He has had a marked decrease in enhancing T1 brain lesions, but continues to accumulate T2 burden on MRI.
Factors to Consider When Assessing Treatment Failure
There are no definitive guidelines for the assessment of treatment failure, so a number of factors should be considered. Significant progression of disability while undergoing therapy is one parameter used to define treatment failure. Because current therapies are only partially effective in slowing the progression of disease, the acceptable amount of disability progression for a specific treatment can be difficult for the neurologist to determine. Progression on the EDSS of 1 or more points is concerning. This patient, for example, had substantial worsening of disability, including development of spasticity, ataxia and bladder dysfunction, fatigue, and depression.
Relapse rate can also help determine treatment failure. If the relapse rate increases to 2 or more episodes a year, the current treatment regimen should be reevaluated. A neurologist may opt not to change therapy for 2 mild attacks in a year, but a change should be considered for 2 or more attacks requiring methylprednisolone intravenously. This patient describes an episode of diplopia 6 months after treatment initiation consistent with a relapse. Because he failed to describe other discrete attacks, he did not fail treatment with relapse rate as a parameter.
MRI is another way to monitor disease activity in MS. Important MRI measures to assess disease activity are the following: (1) T2 lesion number, (2) T2 lesion volume, and (3) gadolinium-enhancing T1 lesions. As described previously, a follow-up MRI scan of this patient's brain with gadolinium showed only 1 enhancing lesion in the left occipital lobe. This radiographic change likely reflects a strong effect of interferon beta-1a on inflammatory change at the blood-brain barrier. In contrast, a significant interval increase in the number of T2-hyperintense lesions from 29 to approximately 40 was seen on FLAIR imaging. His marked increase in T2-hyperintense brain lesions indicates ongoing demyelination over the past 2 years regardless of just 1 enhancing lesion on the follow-up scan.
Therefore, his response to IM interferon beta-1a 30 mcg weekly would be considered a treatment failure because ongoing progression of disability and accumulating T2 MRI disease burden are evident.
Evidence-Based Data for Switching to Another Interferon Medication
A switch to another interferon medication would seem reasonable for this patient because he has been progressing clinically.
Two randomized trials have compared the efficacy of IM interferon-beta-1a 30 mcg weekly with other interferon formulations. The EVIDENCE Trial illustrated the superiority of interferon beta-1a 44 mcg subcutaneous 3 times weekly compared with 30 mcg IM weekly at least in the short term for relapses and MRI lesions.[10,11] The patients were not blinded to the treatment arm, but the evaluating neurologist was blinded. The proportion of patients who were relapse-free at 24 weeks, the primary outcome measure, was 75% with 44 mcg subcutaneously 3 times weekly and 63% with 30 mcg IM weekly (P = .0005). At the end of 48 weeks, 62% of patients on 44 mcg subcutaneously and 52% of patients on 30 mcg IM remained relapse-free (P = .009). The difference between the medications was more pronounced on MRI imaging. Over 48 weeks of the trial, the 44-mcg regimen yielded a 36% decrease in the mean number of T2 lesions per patient per scan, a 38% decrease in the proportion of T2-active scans per patient, and a 32% increase in T2-inactive patients compared with the 30-mcg regimen (all P < .001). There was a trend toward a reduction of disability progression risk, but this trend was not statistically significant. During the 34-week crossover period of the EVIDENCE Trial, patients had 50% fewer relapses (P < .001), and there was a 22% decrease in the mean number of T2-active lesions per patient per scan (P < .02) when patients were switched from 30 mcg IM weekly to 44 mcg subcutaneously 3 times weekly.
The Independent Comparison of Interferon (INCOMIN) trial, performed in Italy, compared interferon beta-1a 30 mcg IM weekly with interferon beta-1b 250 mcg every other day.[12] The trial was randomized, but only the MRI component of the trial was blinded. The percentage of patients who were relapse-free over 2 years was 36% in the interferon beta-1a group and 51% in the interferon beta-1b group (P = .03). Confirmed progression of disability occurred in 30% of the interferon beta-1a patients and in 13% of the interferon beta-1b patients (P = .005). The percentages of patients who were free of new T2 lesions were 26% and 55% in the interferon beta-1a and beta-1b groups, respectively. The percentages of patients who were free of enhancing lesions were 49% on interferon beta-1a and 76% on interferon beta-1b.
Therefore, randomized, clinical trial data have demonstrated advantages of interferon beta-1a 44 mcg 3 times weekly subcutaneously and interferon beta-1b compared with interferon beta-1a 30 mcg weekly IM. The differences in clinical efficacy are more apparent on relapses and MRI activity because the only significant difference on disability progression was nonblinded in the INCOMIN trial.
Evidence-Based Data for Switching to Glatiramer Acetate
A switch to glatiramer acetate is another option to consider for this patient. Because no randomized trial data comparing interferon beta-1a with glatiramer acetate are available, neurologists must rely on the pivotal monotherapy trial data to evaluate the effectiveness of this treatment. A 29% reduction in relapse rate was observed with glatiramer acetate compared with placebo (P = .007) in this trial.[3] In comparison, a 32% reduction in relapse rate was observed with interferon beta-1a compared with placebo (P = .002) in the 172 of 301 patients who finished the 2-year trial. The intention-to-treat analysis of all the interferon beta-1a trial patients demonstrated an 18% reduction in relapse rate over placebo (P = .04).[4] Although direct comparisons across trials can be misleading, glatiramer acetate may be equivalent, if not superior, to interferon beta-1a 30 mcg weekly for relapse reduction.
Although relapse reduction is a goal for this patient, slowing the progression of disability is more critical. Interferon beta-1a demonstrated a 37% reduction in the probability of disease progression over 2 years.[4] In contrast, trial data on reducing the progression of disability with glatiramer acetate are lacking. In the 2-year pivotal trial, 78.4% of patients in the glatiramer acetate group and 75.4% of patients in the placebo group were progression-free (sustained for 90 days). Therefore, in this trial, glatiramer acetate did not significantly slow the progression of disease. On the other hand, a positive effect of glatiramer acetate was seen on nonsustained disability, which was defined as worsening of EDSS ascertained from one exam at one time point in the study.[3] Transient worsening may simply reflect a relapse rather than a true fixed change in disability. Of importance, all the interferon studies relied on confirmed progression that stipulated that worsened disability was still present in a follow-up neurologic exam 3 or 6 months later.
Evidence-Based Data for Switching to Mitoxantrone
Mitoxantrone is another treatment choice for a patient with worsening relapsing-remitting or secondary progressive disease. Administered every 3 months intravenously, mitoxantrone can be a potent agent to reduce relapses, progression of disability, and MRI activity. In a 2-year clinical trial, confirmed progression of disability occurred in 8% of mitoxantrone-treated patients and in 22% of patients in the placebo group. This 62% benefit of treatment was statistically significant (P = .036). The annual exacerbation rate with mitoxantrone was 68% lower than with placebo (P = .001). In addition, the mean number of new T2 MRI lesions was 85% lower than with mitoxantrone (P = .03).[8]
Toxicity risks should be carefully considered before switching this patient to mitoxantrone. Cardiotoxicity can result in a reduced ejection fraction, so new guidelines recommend obtaining echocardiograms or MUltiple Gated Acquisition (MUGA) scans prior to each infusion. The maximum duration of therapy is 2 years, based on cumulative dose. Leukopenia, infection, and rare leukemia can occur. In women, permanent amenorrhea has been reported. Because of the potential risks, trying another immunomodulatory agent first would be reasonable for this patient. If this approach is ineffective, mitoxantrone remains a very effective option.
Evidence for Preventing Progression of Disability Over the Long Term With Treatment
Because a cure for MS has remained elusive, long-term treatment will be important to control this patient's disease. Evidence for reducing the risk of disability progression is an important clinical outcome measure as well as a critical treatment goal for patients.
Interferon Beta-1a 30 mcg and 60 mcg
Interferon beta-1a administered in 30-mcg and 60-mcg weekly IM injections resulted in no difference in disability progression over 4 years.[13] However, no placebo arm was present, so the long-term effect of these regimens on disability is unknown. In the pivotal interferon beta-1a 30-mcg IM weekly trial, the reduction in progression of disability was 37% over 2 years.[4] This result is based on the 172 patients who finished the trial. These 172 patients seem to have responded better to interferon beta-1a because they experienced a 32% reduction in relapse rate, whereas the intention-to-treat analysis of all 301 patients yielded an 18% reduction in relapse rate.
Interferon Beta-1a 44 mcg
Interferon beta-1a 44 mcg given 3 times weekly subcutaneously showed a statistically significant 30% benefit in reducing the risk of disability progression of relapsing-remitting disease at 2 years (P = .014).[5,14] An 8-year follow-up of 382 out of the 560 originally randomized patients examined median time to progression of 1 EDSS point. The median time to progression was 4 years for patients who received placebo for 2 years and then received 44 mcg or 22 mcg of interferon beta-1a for 6 years. In contrast, it took 7.2 years for half the patients to progress in the cohort of patients randomized to the 44-mcg arm for the full 8 years.[15]
Interferon Beta-1b
Interferon beta-1b demonstrated a trend in reduction of disability progression over 5 years in a randomized, double-blind study.[16] The reduction in the probability of disease progression with interferon beta-1b was 23.4%, but this was not statistically significant (P = .096). Although 372 patients entered this trial, only 166 patients were followed in the double-blind cohort to 5 years. Over the first 3 years of the trial, interferon beta-1b also had no significant effect on confirmed progression of disability in relapsing-remitting patients (P = .161).[6] Results from the European secondary progressive trial showed a reduction in the probability of disability progression with interferon beta-1b, whereas results from the North American trial showed no disability benefit with interferon beta-1b.[17]
Glatiramer Acetate
Glatiramer acetate did not demonstrate a significant benefit on disability progression over 10 years with an intent-to-treat analysis.[18] The 59 patients who received glatiramer acetate subcutaneously daily over 10 years experienced a mean increase in EDSS of .9. The 63 patients initially treated with placebo for 36 months and then switched to glatiramer acetate in the open-label phase experienced an increase in EDSS of 1.02. No statistically significant difference was found in this intention-to-treat analysis. Patients who dropped out of the open-label phase of this study had greater disability than the patients remaining on glatiramer acetate over 10 years. Of those patients who dropped out, 29% switched or combined therapies with glatiramer acetate, and 26% perceived their disease as worsening.[19] Therefore, as many as 55% of patients who dropped out were not responding well to glatiramer acetate, which may account for their greater long-term disability.
Slowing the progression of disability over the long term is a major objective for this young man with a lifelong illness. Based on the long-term clinical trial results, initiation of interferon beta-1a 44 mcg subcutaneously 3 times weekly would meet this objective for this patient. Interferon beta-1b might decrease his disability over time, but the trial data for 2 and 5 years were not statistically significant for reduction in disability progression. No long-term evidence on disability progression is available to confirm the use of interferon beta-1a 30 mcg IM weekly and glatiramer acetate subcutaneously weekly.
Add-on Therapies to US Food and Drug Administration-Approved Immunomodulatory Agents for Relapsing-Remitting Disease
Various add-on therapies to US Food and Drug Administration (FDA)-approved immunomodulatory agents are available for relapsing-remitting disease. Because this patient has had increased disability and MRI T2 activity, an add-on medication may be an option to attain better disease control. Add-on therapies may help reduce relapses, disability progression, and MRI activity. However, it should be noted that trial results for many of these agents are limited due to small study sizes.
Methylprednisolone
Zivadinov and colleagues[20] randomized 81 patients with relapsing-remitting disease to receive either 5 days of methylprednisolone with a prednisone taper every 4-6 months, or to receive the same steroid regimen for relapses only. The group that received regular pulse steroid infusions had a 32% reduction in the probability of disability progression compared with the group that received treatment only for relapses (P [21] The risk of developing MS at 2 years was 16.7% with placebo, 14.7% with oral prednisone, and only 7.5% with intravenous methylprednisolone for the treatment of optic neuritis. Adverse effects of steroids are hyperglycemia, avascular necrosis, psychosis, osteoporosis, cataracts, glaucoma, and gastritis.
Methotrexate
There are limited efficacy data for oral weekly methotrexate in the treatment of MS. In a study by Goodkin and colleagues[22] with 60 patients, a significant reduction in disability progression of the upper extremities was observed with 7.5 mg of methotrexate weekly vs placebo over 2 years. In another study by Calabresi and colleagues[23] with 15 patients, methotrexate 20 mg weekly added to interferon beta-1a 30 mcg weekly resulted in a 44% reduction in enhancing lesions over baseline MRI activity compared with interferon beta-1a alone. Risks to consider with methotrexate use are leukopenia, infection, hepatic injury, pulmonary fibrosis, retroperitoneal fibrosis, and secondary malignancies.
Azathioprine
Azathioprine is another oral immunosuppressant therapy with some data to support efficacy in MS. When azathioprine was added to interferon beta-1b in an open-label study at the National Institutes of Health, contrast-enhancing lesions decreased by 69% (P = .002).[24] In addition, a meta-analysis of data from 7 studies revealed better odds for reductions in relapse and EDSS scores with azathioprine therapy.[25] Risks to be considered with adding on azathioprine are leukopenia, infection, hepatic injury, and secondary malignancies.
This patient may benefit from the addition of an add-on medication, such as pulsed intravenous methylprednisolone, oral methotrexate, or oral azathioprine. However, a switch to a higher dose interferon may be a better initial option for him. Risks of these add-on agents must be weighed against the limited efficacy data.
Potential New Treatment Options for This Patient on the Horizon
All of the current FDA-approved medications for this patient have been proven to be only partially effective in the treatment of relapsing-remitting MS. New treatment options are needed so patients like this man can have the best chance of minimizing long-term disability. The 2 main types of new treatments currently being studied in clinical trials are monoclonal antibodies and oral immunomodulatory medications.
Monoclonal Antibodies
Natalizumab. The monoclonal antibody natalizumab was FDA-approved for the treatment of relapsing-remitting MS in November 2004. This antibody binds to an integren on the surface of activated lymphocytes and prevents the lymphocyte from adhering to the vascular cell-adhesion molecule on the endothelial cell. As a result, transendothelial migration that is needed to cause inflammatory activity in the central nervous system cannot occur. In the 2-year trial, natalizumab reduced relapses by 68% (P < .001) and reduced the probability of disability progression (P = .002) compared with placebo.[26] Treatment with natalizumab was associated with an 83% reduction in new or enlarged T2 MRI lesions (P < .0001), a 92% reduction in gadolinium-enhancing lesions (P < .0001), and 76% fewer new black holes.[27]
Biogen voluntarily withdrew natalizumab from the marketplace in February 2005 after 2 cases of progressive multifocal leukoencephalopathy developed in MS patients receiving therapy in combination with interferon beta-1a 30 mcg IM weekly. A subsequent case was retrospectively discovered in a Crohn's patient who died while receiving treatment with natalizumab.
Monoclonal antibodies in development. Daclizumab is a promising monoclonal antibody therapy directed against the interleukin (IL)-2 receptor. Anti-CTLA4 antibodies, which disrupt T cells from interacting with antigen-presenting cells, are also being investigated in preliminary studies.[28] Monoclonal antibodies that are directed against the proinflammatory cytokines IL-12 and IL-23 and the B-cell surface marker CD20 are also being studied.
Oral Immunomodulatory Medications
Oral agents with preliminary encouraging results in phase 1 or phase 2 clinical trials are cladribine, fumarate, laquinimod, and statins. These agents have immunosuppressive or immunodulatory properties that may potentially influence the disease course in MS.
Conclusion
This young man with MS has several treatment options to change the natural history of his disease. Ideally, the treatment selected will reduce his relapses, slow the progression of his disability, and decrease his MRI activity. Because current therapies are only partially effective, knowing when to change treatments is sometimes difficult. Neurologists must rely on pivotal monotherapy trials, randomized comparison trials, and limited add-on agent studies to choose the next treatment plan when current treatment fails. New options, such as monoclonal antibodies and oral immunomodulatory medications, will likely continue to advance the treatment of MS.
References
- Paty DW, Ebers GC. Multiple Sclerosis. Philadelphia, Pa: FA Davis; 1998.
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112:133-146. Abstract
- Johnson KP, Brooks BR, Cohen JA. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45:1268-1276. Abstract
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285-294. Abstract
- PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498-1504. Abstract
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655-661. Abstract
- Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528-1532. Abstract
- Hartung HP, Gonsette R, Konig K, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomized, multicentre trial. Lancet. 2002;360:2018-2025. Abstract
- Scott TF, Schramke CJ, Novero J, et al. Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology. 2000;55:689-693. Abstract
- Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology. 2002;59:1496-1506. Abstract
- Coyle P, Goodin D, O'Connor P, et al. The EVIDENCE study crossover. Program and abstracts of the 13th Meeting of the European Neurological Society; June 14-18, 2003; Istanbul, Turkey.
- Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomized multicentre study (INCOMIN). Lancet. 2002;359:1453-1460. Abstract
- Clanet M, Radue EW, Kappos L, et al. A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology. 2002;59:1507-1517. Abstract
- Gooden DS, Frohman EM, Garmany GP, et al. Disease modifying therapies in multiple sclerosis. Neurology. 2002;58:169-178. Abstract
- Paty D, Kappos L, Moraga MS, et al. Long-term observation efficacy and safety follow-up of the PRISMS cohort. Program and abstracts of the European Committee for Treatment and Research in Multiple Sclerosis; September 17-20, 2003; Milan, Italy.
- IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277-1285. Abstract
- European Study Group on Interferon beta-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491-1497. Abstract
- Ford C, Johnson K, Brooks B, et al. Sustained efficacy and tolerability of glatiramer acetate in relapsing-remitting multiple sclerosis patients for over 10 years. Program and abstracts of the European Committee for Treatment and Research in Multiple Sclerosis; September 17-20, 2003; Milan, Italy.
- Johnson KP, Panitch HS, Ford CC, Lisak RP; Copaxone Study Group. Longterm slowing of disability progression in patients receiving continuous glatiramer acetate compared with those withdrawing from therapy: 10 year results from an ongoing trial. Program and abstracts of the American Academy of Neurology Meeting; April 24-May 1, 2004; San Francisco, California. Abstract S20.004.
- Zivadinov R, Rudick RA, De Masi R, et al. Effects of IV methylprednisolone on brain atrophy in relapsing-remitting MS. Neurology. 2001;57:1239-1247. Abstract
- Beck RW, Cleary PA, Trobe JD, et al. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. The Optic Neuritis Study Group. N Engl J Med. 1993;329:1764-1769. Abstract
- Goodkin DE, Rudick RA, VanderBrug Medendorp S, et al. Low-dose oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol. 1995;37:30-40. Abstract
- Calabresi PA, Wilterdink JL, Rogg JM, et al. An open-label trial of combination therapy with interferon beta-1a and oral methotrexate in MS. Neurology. 2002;58:314-317. Abstract
- Markovic-Plese S, Bielekova B, Kadom N, et al. Longitudinal MRI study: the effects of azathioprine in MS patients refractory to interferon beta-1b. Neurology. 2003;60:1849-1851. Abstract
- Yudkin PL, Ellison GW, Ghezzi A, et al. Overview of azathioprine treatment in multiple sclerosis. Lancet. 1991;338:1051-1055. Abstract
- Polman C, O'Connor P, Havrdova E, et al. Clinical results from AFFIRM: a randomized, double-blind, placebo-controlled, multicenter trial to determine the efficacy and safety of natalizumab in patients with relapsing multiple sclerosis. Program and abstracts of the American Academy of Neurology 57th Annual Meeting; April 9-16, 2005; Miami Beach, Florida. Abstract S16.003.
- Miller D, O'Connor P, Havrdova E, et al. The efficacy of natalizumab on MRI measures in patients with relapsing multiple sclerosis: results from the AFFIRM trial. Program and abstracts of the American Academy of Neurology 57th Annual Meeting; April 9-16, 2005; Miami Beach, Florida. Abstract S16.005.
- Khoury J, Viglietta V, Buckle G, et al. A phase I trial of CTLA41g treatment in MS. Program and abstracts of the American Academy of Neurology 57th Annual Meeting; April 9-16, 2005; Miami Beach, Florida.
Authors and Disclosures
As an organization accredited by the ACCME, Medscape requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines "relevant financial relationships" as "financial relationships in any amount, occurring within the past 12 months, that create a conflict of interest."
Medscape encourages Authors to identify investigational products or off-label uses of products regulated by the U.S. Food and Drug Administration, at first mention and where appropriate in the content.
Author
Barry A. Singer, MD
Assistant Professor of Clinical Neurology, Washington University School of Medicine, St. Louis, Missouri
Disclosure: Barry A. Singer, MD, has disclosed that he has received grants for clinical research from Serono and Pfizer and grants for educational activities from Biogen. Dr. Singer has also disclosed that he has served as an advisor or consultant to Serono, Pfizer, Berlex, Biogen, and Teva Neuroscience.
|
Editor
Marni Kelman, MSc
Program Director, Medscape Neurology & Neurosurgery
Disclosure: Marni Kelman has disclosed no relevant financial relationships.
|











